You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
The Official Homework Help Thread
- Thread starter Zandy
- Start date
Acruoxil
Senior Member
Best way to remember all 206 bones, the sutures and foramens? Fun ideas or tricks?
As a Biology student, I just stick notes on my wall on the places I frequent the most in my room: beside my bed, right next to my PC/Study table. Basically places my eyeballs frequently dart over. It helps me memorize very well
Last edited:
YearsLate
Considerate Cynic
As a Biology students, I just stick notes on my wall on the places I frequent the most in my room: beside my bed, right next to my PC/Study table. Basically places my eyeballs frequently dart over. It helps me memorize very well
I would endorse doing this, as well as also saying the name of some of those bones. Speaking them can help embed them in your memory. Just don't try to say them all at the same time, you'll end up making it into a chore instead of a few things to cement in your memory.
if someone could let me know if this is correct it would be greatly appreciated c:
On doit porter des v?tements sp?ciaux tel que un combinaison de plong?e = we (?) must wear special clothes such as a diving suit
also, how would you say 'you need' as in you need special equipment? Thank you in advance ^^
On doit porter des v?tements sp?ciaux tel que un combinaison de plong?e = we (?) must wear special clothes such as a diving suit
also, how would you say 'you need' as in you need special equipment? Thank you in advance ^^
AnonymousFish
lovable scrub
if someone could let me know if this is correct it would be greatly appreciated c:
On doit porter des v?tements sp?ciaux tel que un combinaison de plong?e = we (?) must wear special clothes such as a diving suit
also, how would you say 'you need' as in you need special equipment? Thank you in advance ^^
I think that's right, but I'm not 100% sure... ^^;
As for "you need," you could use the subjunctive if you know it... as in, "It's necessary that you get special equipment" (Il faut que tu obtiennes/vous obteniez...)
Bonne chance! J'espere que ca te servira >.<
thanks for the tips! however, my test will be basically arrows pointing to the spot and then i have to remember the name. did your sticky notes have the bone picture as well? how did the word alone on a note help you remember the location? so far learning the latin meaning of some of the bones has helped me but some of the bones and holes look exactly alike ;.;I would endorse doing this, as well as also saying the name of some of those bones. Speaking them can help embed them in your memory. Just don't try to say them all at the same time, you'll end up making it into a chore instead of a few things to cement in your memory.
I think that's right, but I'm not 100% sure... ^^;
As for "you need," you could use the subjunctive if you know it... as in, "It's necessary that you get special equipment" (Il faut que tu obtiennes/vous obteniez...)
Bonne chance! J'espere que ca te servira >.<
Oh ok thank you~
Wholockian
Midochlorians are the power house of the force
Ok, so I have a question to do, that I really can't make any sense of:
"Next to each of the above words, write which part of speech it is, as used in the passage"
What exactly is it asking me to do?
"Next to each of the above words, write which part of speech it is, as used in the passage"
What exactly is it asking me to do?
MelaniteMoon
Egg(?)
Ok, so I have a question to do, that I really can't make any sense of:
"Next to each of the above words, write which part of speech it is, as used in the passage"
What exactly is it asking me to do?
Did you have a paragraph or story to read beforehand?
A part of speech is the type of word that the word is (ex: noun, adjective, verb, etc.) but different words can be different types depending on the context of the sentence, (ex: I caught a fish (noun), I fish every year (verb)) so it should be asking you to choose which type it is depending on the context of the sentence.
heyo i thought i may as well ask here, i'm having a bit of trouble w/ chemistry homework and idk what i'm doing wrong!!
the question asks: A gas sample contains 16.0g of CH4, 16.0g of O2, 16.0g of SO2, and 33.0g of CO2. What is the total number of moles of gas in the sample?
the way i've worked it out i got 2.99 total moles, but the correct answer is actually 2.50 moles.
16.0g CH4 (1 mol/ 16.042g) = 0.997 mol
16.0g O2 (1 mol/16.00g) = 1 mol WOW ok lame i used the molar mass of 1 oxygen instead of 2 NEVERMIND
16.0g SO2 (1 mol/64.06g) = 0.25 mol
33.0g CO2 (1 mol/44.01g) = 0.750 mol
what??? am??? i??? missing??? nvm i'm rly dumb
the question asks: A gas sample contains 16.0g of CH4, 16.0g of O2, 16.0g of SO2, and 33.0g of CO2. What is the total number of moles of gas in the sample?
the way i've worked it out i got 2.99 total moles, but the correct answer is actually 2.50 moles.
16.0g CH4 (1 mol/ 16.042g) = 0.997 mol
16.0g SO2 (1 mol/64.06g) = 0.25 mol
33.0g CO2 (1 mol/44.01g) = 0.750 mol
Last edited:
JellyLu
🌒🌓🌔🌕🌖🌗🌘
I need help with organic chemistry ;;
My professor keeps referring to the amount pi orbitals a molecule (i.e. benzene) has and I just can't seem to understand what he means. He draws diagrams putting electrons orbitals labeled "pi1, pi2" all the way up to things like "pi4*" or "pi6*"><
Can someone please explain to me how to determine the amount of pi orbitals something has and what exactly the "*" means? I'm sorry if this is a dumb question but any help would be appreciated ;;
My professor keeps referring to the amount pi orbitals a molecule (i.e. benzene) has and I just can't seem to understand what he means. He draws diagrams putting electrons orbitals labeled "pi1, pi2" all the way up to things like "pi4*" or "pi6*"><
Can someone please explain to me how to determine the amount of pi orbitals something has and what exactly the "*" means? I'm sorry if this is a dumb question but any help would be appreciated ;;
I need help with organic chemistry ;;
My professor keeps referring to the amount pi orbitals a molecule (i.e. benzene) has and I just can't seem to understand what he means. He draws diagrams putting electrons orbitals labeled "pi1, pi2" all the way up to things like "pi4*" or "pi6*"><
Can someone please explain to me how to determine the amount of pi orbitals something has and what exactly the "*" means? I'm sorry if this is a dumb question but any help would be appreciated ;;
Generally the * refers to the nonbonding orbitals in molecular orbital theory.
Here's a ridiculously large image of the MO diagram for NO:
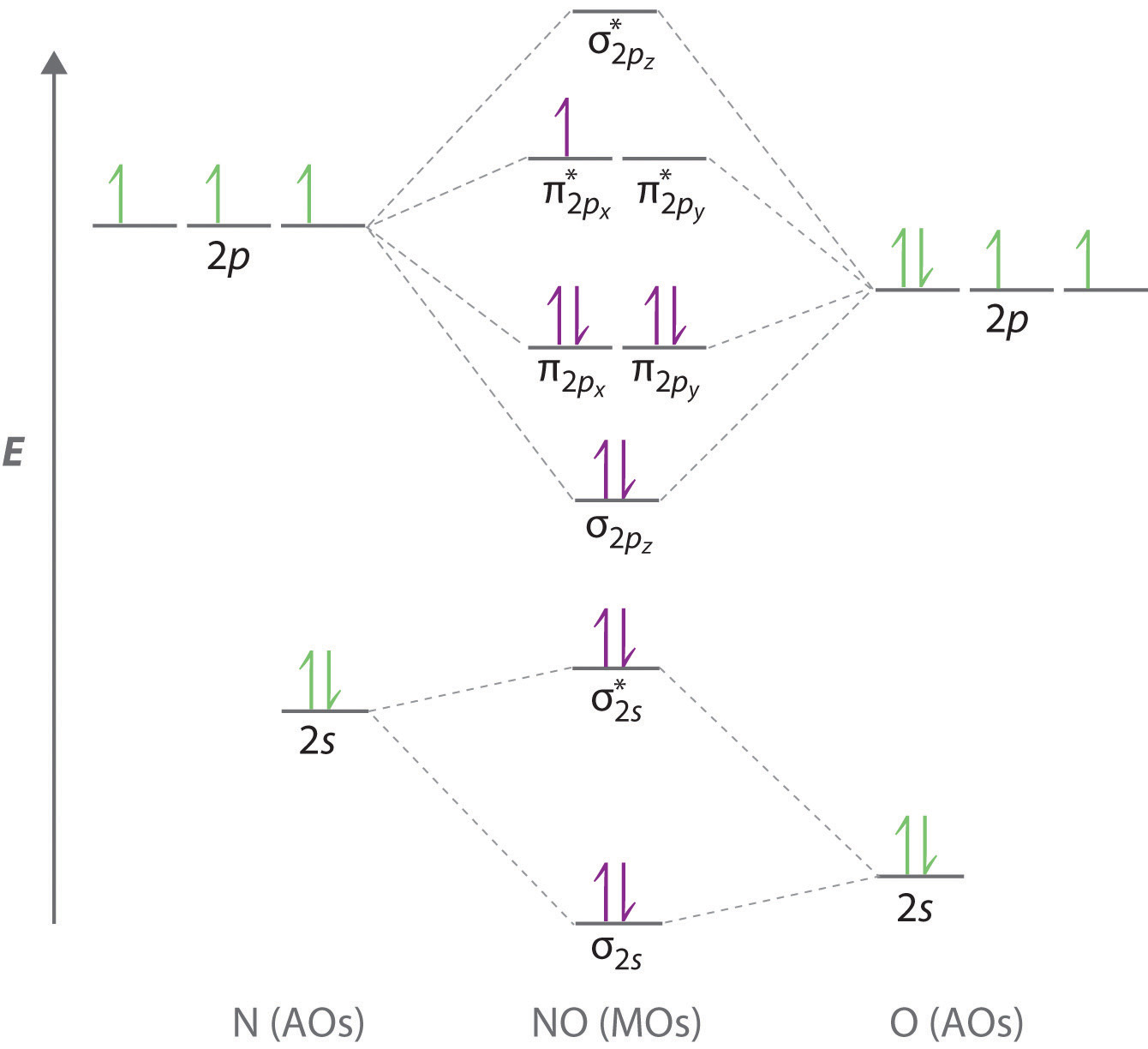
So basically in a given molecule there are as many molecular orbitals as there are atomic orbitals (in the sum of the constituent atoms). The orbitals can be sigma (lower energy) or pi (higher energy). Each of these can be subcategorized as bonding and non-bonding (*) orbitals. Honestly when I took chem we just memorized the MO diagrams since there are only really 2 different versions used in organic.
That being said I'd normally just use hybridization theory to solve the problem. If you're asked how many pi electrons are in benzene, it would just be the electrons in the pi bonds, so 6. In something that has lone pairs, one lone pair from each atom without a double bond counts in the pi system. For example:
.jpg)
This molecule has 2 double bonds (2 pi bonds so 4 pi electrons) as well as a lone pair on the oxygen that is perpendicular to the plane of the ring (in a p orbital), so a total of 6 pi electrons. The second lone pair of the oxygen does not count since it is in a hybrid sp2 orbital that is parallel to the plane of the ring.
Hope that wasn't too convoluted, let me know if you need clarification!
Last edited:
JellyLu
🌒🌓🌔🌕🌖🌗🌘
Generally the * refers to the nonbonding orbitals in molecular orbital theory.
Here's a ridiculously large image of the MO diagram for NO:

So basically in a given molecule there are as many molecular orbitals as there are atomic orbitals (in the sum of the constituent atoms). The orbitals can be sigma (lower energy) or pi (higher energy). Each of these can be subcategorized as bonding and non-bonding (*) orbitals. Honestly when I took chem we just memorized the MO diagrams since there are only really 2 different versions used in organic.
That being said I'd normally just use hybridization theory to solve the problem. If you're asked how many pi electrons are in benzene, it would just be the electrons in the pi bonds, so 6. In something that has lone pairs, one lone pair from each atom without a double bond counts in the pi system. For example:
.jpg)
This molecule has 2 double bonds (2 pi bonds so 4 pi electrons) as well as a lone pair on the oxygen that is perpendicular to the plane of the ring (in a p orbital), so a total of 6 pi electrons. The second lone pair of the oxygen does not count since it is in a hybrid sp2 orbital that is parallel to the plane of the ring.
Hope that wasn't too convoluted, let me know if you need clarification!
Oh goodness! Thank you so much! This really helped me
EDIT: Sorry for the additional questions ^^; but would you also happen to have an expanation for when a molecule contains a N?
Last edited:
Oh goodness! Thank you so much! This really helped meMy professor kept giving an equation to find the number of pi electrons, 4n+2 or something. Do you know how that relates? o:
EDIT: Sorry for the additional questions ^^; but would you also happen to have an expanation for when a molecule contains a N?
Yeah for sure! So 4n + 2 is the rule for aromaticity. For example if n=1, 4(1) +2 = 6 so that means that a compound with 6 pi electrons is aromatic. Similarly a compound with 10 pi electrons [4(2) +2 = 10] would also be aromatic. There are also other requirements but that rule is a good start. I'll give you a couple examples with nitrogen.

So in pyridine, there are 6 pi electrons. This is because a single atom can only contribute to the pi system with a bond or a lone pair, not both. So the pi bond electrons count, but the lone pair is in a hybrid sp2 and does not. Since 4(1) +2 = 6, pyridine is aromatic.
With pyrrole however, the lone pair on the N does count since there are no double bonds attached to it. So it has 6 pi electrons and is also aromatic. The last 2 examples are like the one I already showed you - only one lone pair from the O and S are in p orbitals and are therefore counted as pi electrons.
JellyLu
🌒🌓🌔🌕🌖🌗🌘
Yeah for sure! So 4n + 2 is the rule for aromaticity. For example if n=1, 4(1) +2 = 6 so that means that a compound with 6 pi electrons is aromatic. Similarly a compound with 10 pi electrons [4(2) +2 = 10] would also be aromatic. There are also other requirements but that rule is a good start. I'll give you a couple examples with nitrogen.

So in pyridine, there are 6 pi electrons. This is because a single atom can only contribute to the pi system with a bond or a lone pair, not both. So the pi bond electrons count, but the lone pair is in a hybrid sp2 and does not. Since 4(1) +2 = 6, pyridine is aromatic.
With pyrrole however, the lone pair on the N does count since there are no double bonds attached to it. So it has 6 pi electrons and is also aromatic. The last 2 examples are like the one I already showed you - only one lone pair from the O and S are in p orbitals and are therefore counted as pi electrons.
Thank you so much! You made everything so much more understandable
Also , sorry again if I was a bother ^^; I know the questions I asked were kinda silly
Last edited:
Similar threads
- Replies
- 6
- Views
- 1K
- Replies
- 0
- Views
- 407